
SL Paper 2
Boron is most often encountered as a component in borosilicate glass (heat resistant glass).
The naturally occurring element contains two stable isotopes, \(_{\;{\text{5}}}^{{\text{10}}}{\text{B}}\) and \(_{\;{\text{5}}}^{{\text{11}}}{\text{B}}\).
State the number of protons, neutrons and electrons in an atom of \(_{\;{\text{5}}}^{{\text{11}}}{\text{B}}\).

The relative atomic mass of boron is 10.8, to three significant figures. Calculate the percentage of \(_{\;{\text{5}}}^{{\text{10}}}{\text{B}}\) in the naturally occurring element.
Isotopes of boron containing 7 and 8 neutrons also exist. Suggest why releasing isotopes containing more neutrons than the stable isotope into the environment can be dangerous.
(i) State the formula of the compound that boron forms with fluorine.
(ii) Explain why this compound acts as a Lewis acid.
Ammonia, \({\text{N}}{{\text{H}}_{\text{3}}}\), is a base according to both the Brønsted–Lowry and the Lewis theories of acids and bases.
The equation for the reaction between sodium hydroxide, NaOH, and nitric acid, \({\text{HN}}{{\text{O}}_{\text{3}}}\), is shown below.
\[\begin{array}{*{20}{l}} {{\text{NaOH(aq)}} + {\text{HN}}{{\text{O}}_3}{\text{(aq)}} \to {\text{NaN}}{{\text{O}}_3}{\text{(aq)}} + {{\text{H}}_2}{\text{O(l)}}}&{{\text{ }}\Delta H = - 57.6{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}} \end{array}\]
Distinguish between the terms strong base and weak base, and state one example of each.
State the equation for the reaction of ammonia with water.
Explain why ammonia can act as a Brønsted–Lowry base.
Explain why ammonia can also act as a Lewis base.
(i) When ammonium chloride, \({\text{N}}{{\text{H}}_{\text{4}}}{\text{Cl(aq)}}\), is added to excess solid sodium carbonate, \({\text{N}}{{\text{a}}_{\text{2}}}{\text{C}}{{\text{O}}_{\text{3}}}{\text{(s)}}\), an acid–base reaction occurs. Bubbles of gas are produced and the solid sodium carbonate decreases in mass. State one difference which would be observed if nitric acid, \({\text{HN}}{{\text{O}}_{\text{3}}}{\text{(aq)}}\), was used instead of ammonium chloride.
(ii) Deduce the Lewis structures of the ammonium ion, \({\text{NH}}_4^ + \), and the carbonate ion, \({\text{CO}}_3^{2 - }\).
Ammonium ion\(\quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \)Carbonate ion
(iii) Predict the shapes of \({\text{NH}}_4^ + \) and \({\text{CO}}_3^{2 - }\).
\({\text{NH}}_4^ + \):
\({\text{CO}}_3^{2 - }\):
(i) Sketch and label an enthalpy level diagram for this reaction.
(ii) Deduce whether the reactants or the products are more energetically stable, stating your reasoning.
(iii) Calculate the change in heat energy, in kJ, when \({\text{50.0 c}}{{\text{m}}^{\text{3}}}\) of \({\text{2.50 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sodium hydroxide solution is added to excess nitric acid.
When 5.35 g ammonium chloride, \({\text{N}}{{\text{H}}_{\text{4}}}{\text{Cl(s)}}\), is added to \({\text{100.0 c}}{{\text{m}}^{\text{3}}}\) of water, the temperature of the water decreases from 19.30 °C to 15.80 °C. Determine the enthalpy change, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), for the dissolving of ammonium chloride in water.
When nitrogen gas and hydrogen gas are allowed to react in a closed container, the following equilibrium is established.
\[{{\text{N}}_{\text{2}}}{\text{(g)}} + {\text{3}}{{\text{H}}_{\text{2}}}{\text{(g)}} \rightleftharpoons {\text{2N}}{{\text{H}}_{\text{3}}}{\text{(g)}}\;\;\;\;\;\Delta H = - 92.6{\text{ kJ}}\]
Outline two characteristics of a reversible reaction in a state of dynamic equilibrium.
Deduce the equilibrium constant expression, \({K_{\text{c}}}\), for the reaction.
Predict, with a reason, how each of the following changes affects the position of equilibrium.
The volume of the container is increased.
Ammonia is removed from the equilibrium mixture.
Define the term activation energy, \({E_{\text{a}}}\).
Ammonia is manufactured by the Haber process in which iron is used as a catalyst. Explain the effect of a catalyst on the rate of reaction.
Sketch the Maxwell–Boltzmann energy distribution curve for a reaction, labelling both axes and showing the activation energy with and without a catalyst.
Typical conditions used in the Haber process are 500 °C and 200 atm, resulting in approximately 15% yield of ammonia.
(i) Explain why a temperature lower than 500 °C is not used.
(ii) Outline why a pressure higher than 200 atm is not often used.
Define the term base according to the Lewis theory.
Define the term weak base according to the Brønsted-Lowry theory.
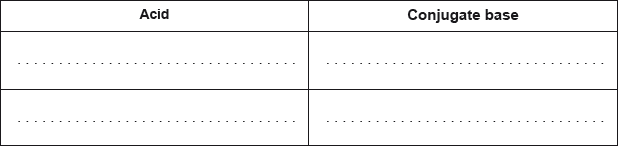
Deduce the formulas of conjugate acid-base pairs in the reaction below.
\[{\text{C}}{{\text{H}}_{\text{3}}}{\text{N}}{{\text{H}}_{\text{2}}}{\text{(aq)}} + {{\text{H}}_{\text{2}}}{\text{O(l)}} \rightleftharpoons {\text{C}}{{\text{H}}_{\text{3}}}{\text{NH}}_{\text{3}}^ + {\text{(aq)}} + {\text{O}}{{\text{H}}^ - }{\text{(aq)}}\]
Outline an experiment and its results which could be used to distinguish between a strong base and a weak base.
Across period 3, elements increase in atomic number, decrease in atomic radius and increase in electronegativity.
Define the term electronegativity.
Explain why the atomic radius of elements decreases across the period.
State the equations for the reactions of sodium oxide with water and phosphorus(V) oxide with water.
Suggest the pH of the solutions formed in part (c) (i).
Describe three tests that can be carried out in the laboratory, and the expected results, to distinguish between \({\text{0.10 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{ HCl(aq)}}\) and \({\text{0.10 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{ C}}{{\text{H}}_{\text{3}}}{\text{COOH(aq)}}\).
Explain whether BF3 can act as a Brønsted-Lowry acid, a Lewis acid or both.
Describe the bonding and structure of sodium chloride.
State the formula of the compounds formed between the elements below.
Sodium and sulfur:
Magnesium and phosphorus:
Covalent bonds form when phosphorus reacts with chlorine to form \({\text{PC}}{{\text{l}}_{\text{3}}}\). Deduce the Lewis (electron dot) structure, the shape and bond angle in \({\text{PC}}{{\text{l}}_{\text{3}}}\) and explain why the molecule is polar.
Lewis (electron dot) structure:
Name of shape:
Bond angle:
Explanation of polarity of molecule: